University of 91Ö±˛Ą researchers have used cryo-electron microscopy to determine the structures of two key photosynthetic complexes from a purple bacterium, providing new insights into how the absorption of light is wired to the storage of energy in chemical forms.
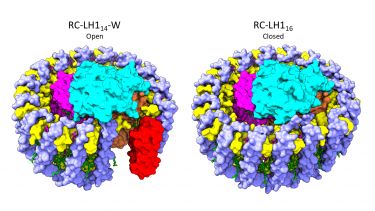
Purple phototropic bacteria contain reaction centres, which use light to power a series of electron transfers that convert light energy into chemical energy. Each reaction centre (RC) is surrounded by a light-harvesting antenna called LH1, which greatly increases the amount of light that can be used for this process. Despite being formed from a set of common building blocks, LH1 complexes are remarkably diverse; the simplest such antenna is formed from 16 subunits in a closed ring surrounding the RC whilst others comprise 14 or 15 subunits in an open ring. Scientists in the group of Professor Neil Hunter FRS in the Department of Molecular Biology and Biotechnology have used cryo-electron microscopy to determine the structures of the RC-LH1 complexes from Rhodopseudomonas palustris, which produces both open and closed LH1 rings. These two structures provide a unique insight into the evolution of RC-LH1 complexes and how they are wired to other energy generating components in the photosynthetic cell.
By studying two RC-LH1 complexes from a single species, we can better understand how evolution has balanced the absorption of photons with the need to electrically connect reaction centres to nearby proteins that complete the process of energy storage.
Dr David Swainsbury
Lead author
The lead author of the study, Dr David Swainsbury, said “Our structures provide a new view of bacterial photosynthesis and provide a valuable insight into the more complicated process found in plants and algae. By studying two RC-LH1 complexes from a single species, we can better understand how evolution has balanced the absorption of photons with the need to electrically connect reaction centres to nearby proteins that complete the process of energy storage. Our findings have great potential for engineering these bacteria to improve photosynthesis in this and other organisms, providing more green energy to produce biofuel and bio-hydrogen.”
The two RC-LH1 structures also reveal a previously unobserved conformational change within the reaction centre protein, where the energy of light is converted to an electrical charge. Another author of the study, Dr Andrew Hitchcock, added “This observation provides new insight into the function of this critical light-driven enzyme, which is evolutionarily related to the photosystem II complex in plants and algae that produces all of the Earth’s oxygen. Further work is required to fully understand this conformational switch and other dynamic structural changes that occur when bacteria and plants absorb and store solar energy”.
The study was published in Science Advances on 13th January 2021. The research was conducted in collaboration with researchers at Washington University in St. Louis (USA), the Astbury Centre for Structural and Molecular Biology at the University of Leeds, and the Institute of Systems, Molecular and Integrative Biology at the University of Liverpool.